Experimental protocol for detecting changes in intracellular pH by the FLIPR TETRA system
Introduction
The FLIPR TETRA system can be used to perform most cell-based experimental studies. This application study recommends a basic protocol for the operation of intracellular pH detection experiments on FLIPR . The experiment was done on adherent cells, and optimization of some important parameters was also discussed. Because each cell line has its own unique characteristics, each researcher needs to be individually optimized for his or her own experiments.
Principle of intracellular pH experiment
The Na + /H + exchanger ( NHE ) discharges hydrogen ions while pumping sodium ions to maintain pH stability. BCECF is a pH sensitive dye which has a higher absorbance under alkaline conditions and a lower pH under acidic conditions. Thus BCECF can be used to monitor intracellular pH changes to reflect NHE activity. Cells were incubated BCECF was added, followed by addition of NH 4 Cl, NH 4 Cl help promote the dye into the cell, and NH 3 causes alkaline environment (high fluorescence signal). When NH 4 Cl is diluted by the FLIPR- added solution, NH 3 diffuses out of the cell, causing an increase in the intracellular H + concentration, which in turn causes a rapid decrease in the fluorescent signal. These H + will be pumped out of the cells by the NHE (slow rise in fluorescence signal is relatively corresponding to NHE activity).
Cell preparation
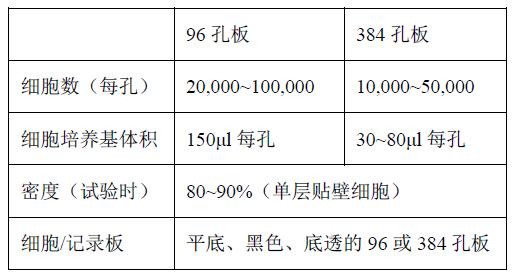
Cells were seeded in multiwell plates one day prior to the experiment, and all subsequent steps were performed in 96- well plates or 384- well plates. The specific steps are as follows:
1. Seed cells in 96 or 384 well plates
2. Load BCECF into the cells for 45 minutes.
3. Add 20 mM NH 4 Cl to the cell plate to acidify the cells and incubate for 15 minutes.
4. Wash the cells with buffer containing 20 mM NH 4 Cl
5, using FLIPR for detection; NH 4 Cl buffer is diluted with a large volume of balanced salt solution and various ligands / controls.
It is necessary to optimize the density of the cell seed plate to ensure that a uniform monolayer of similar density can be formed per well after overnight culture. By appropriately adjusting the density of the seed plates, the cells can be cultured for more than one day to meet the requirements of the day of the experiment. Cells with weak adhesion or irregular growth need to be cultured in multi-well plates coated with polylysine, laminin or collagen.
Dye loading
In order to observe changes in pH within the cells, it is necessary to load pH- sensitive fluorescent dyes into the cells . The dye loading protocol of different cell types needs to be optimized.
Fluorescent dyes
To date, BCECF is the most widely used fluorescent dye reagent for intracellular pH detection experiments.
Anion exchanger inhibitor
Some types of cells transfer anions to the outside of the cell via anion exchange proteins, which contain fluorescent dyes. This not only leads to a weakening of the dye loading effect, but also to the error of the final data result; when the compound to be detected is added, the recorded fluorescent signal value is drastically reduced due to the dilution of the extracellular dye. (The compound to be tested is diluted with a dye-free buffer) Thus, inhibition of anion exchanger activity is key to the successful detection of FLIPR .
Probenecid is an anion exchange protein inhibitor that, when added to a loading medium, increases the proportion of dye retained in the cell. One cell type known to require probenecid is CHO . Although probenecid can delay the transfer of dye from the cell to the extracellular, it is somewhat toxic to cells, so the incubation time after dye loading should be controlled as much as possible in the shortest possible time. .
Piperazine is another anion exchange protein inhibitor. To date, little information has been used on phenylsulfazolone in intracellular pH assays.
Medium for dye loading
Several types of dye loading cultures were tested. The best medium should be able to balance both aspects - dye loading and good cellular response. Possible choices are:
1. Growth medium + 10% fetal bovine serum (FBS) + 20 mM HEPES
2. Growth medium + 1% FBS + 20 mM HEPES
3. Hank ' s BSS 1X (with no probenec) + 20 mM HEPES +1% FBS
4. Hank ' s BSS 1X (with no probenecid) + 20 mM HEPES +1% BSA
• Incubation time and temperature after dye loading
The optimal incubation time depends on the cell type. Since fluorescent dyes are toxic to cells, the incubation time is strictly controlled. 60 minutes, 37 ° C is an incubation condition that is generally effective for most cells, and is generally the initial recommended condition for method development.
Note: If an acceptable fluorescent signal is obtained by incubating for 30 minutes, a shorter incubation period is recommended.
Loading of acidic solution in cells Loading of acidic solution in cells
This step is not to add the acid itself directly, but to add NH 4 Cl to the cells . When NH4Cl and NH 3 formed in the cell cytoplasm was basified, then when the diluted solution is added FLIPR system 4 Cl NH, NH 3 diffusion out of the cell such that the high concentration of residual intracellular H +. This will stimulate the activity of NHE and expel hydrogen ions out of the cell.
The solution used in this step was 200 mM NH 4 Cl . NH 4 Cl was kept in the multiwell plate until the FLIPR addition solution was allowed to be diluted.
Compound plate preparation
In this experiment, since the incubation time after the dye loading was long enough, it was easy to prepare the desired compound plate.
Prepare compound dilution buffer
In the intracellular pH assay, the buffer used to dilute the compound and the buffer to wash the cells were different (compared to intracellular calcium flux and membrane potential experiments). The wash buffer contains 20 mM NH4Cl and the compound dilution buffer does not contain NH4Cl . The compound to be tested should be diluted to 1.25 times the final concentration at the time of the experiment .
Cell cleaning
After the dye was loaded and the NH 4 Cl solution was added, it was washed several times with a washing buffer to remove the extracellular dye. 20 mM NH 4 Cl is required for all washing steps . Company recommends using a Molecular Devices microplate washer for 96- or 384-well plates, and the rate of adjustable height.
The high quality data obtained from the FLIPR system depends in part on the performance of the washing machine in the cell cleaning stage, and whether the remaining volume in each well after the last cleaning is consistent is guaranteed. The results obtained after manual cleaning are more different. In addition, if manual cleaning is important, be careful not to blot the solution in the well between the two washing steps so that the cells are directly exposed to the air.
Note : The buffer for washing buffer and diluted compound is not the same.
Running the experiment
Plant cells in the day before the experiment Plant cells in the day before the experiment Plant cells in the day before the experiment Plant cells in the day before the experiment Plant cells in the day before the experiment Corresponding black wall Corresponding black wall Corresponding black wall 96 or 384 well cell plate :
1 ) Add BCECF and incubate for 45 minutes
2 ) Add 20mM NH 4 Cl, incubated , incubate for 15 minutes
3 ) Wash cells with buffer containing 20 mM NH 4 Cl
4 ) Start experiment
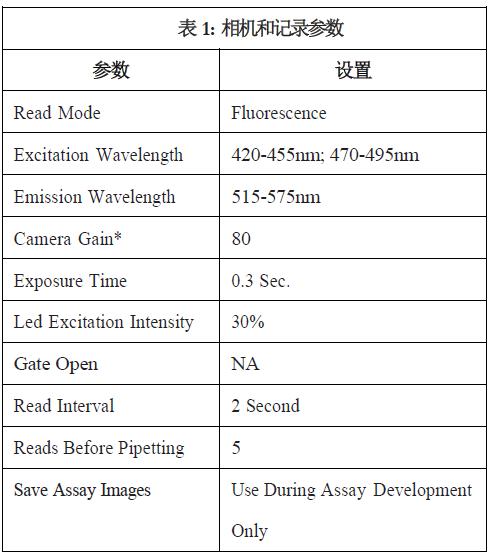
Instrument settings
The parameters set by the FLIPR system in the table below apply to the BCECF experiments for all CHO cells .
Roundness Measuring Instrument
Roundness Measuring Instrument,Roundness Tester Machine,Mitutoyo Roundness Tester,Roundness Tester Price
Zhejiang dexun instrument technology co., ltd , https://www.dexunmeasuring.com
