
The famous 19th-century doctor William Osler once said: "Good doctors treat diseases, and great doctors treat patients who are ill." But in the 20th century, the medical community usually regarded patients as a collection of symptoms rather than an individual. This situation is exacerbated by the development of the medical service system, which makes the patient's care fragmented.
Digital health has the potential to make Dr. William Osler's patient-centric vision of care a reality. Digital health tools have enormous potential to improve our ability to accurately diagnose and treat diseases, and to strengthen our personal care, truly patient-centric.
We tend to overestimate the short-term effects of a technology but underestimate its long-term effects. In my opinion, the future of digital health is now. The FDA is taking some new steps to ensure it benefits. Most notably, we are expanding the opportunities for digital medical tools as part of drug review and combining these capabilities with drug delivery to form drug delivery systems.
We are expanding the pre-marketing review of digital health tools as a medical device through a new pre-certification program. We are implementing a new approach to reviewing artificial intelligence. We also announced a new digital health tool to apply to our own work – pre-market review of drug safety. Finally, we are launching a new digital health incubator. I will talk about these initiatives in turn and how they build on our broader perspective.
Strong digital health technologies have been promoted, and other technologies will be promoted in the next few years. For example, mobile health apps (apps) are already helping consumers manage their health by better controlling their diet and lifestyle. More and more mobile health apps are showing promise in areas such as diabetes prevention, asthma and addiction recovery. According to industry estimates, by the end of this year, 50% of smartphone and tablet users in the US will download mobile health apps.
In addition to empowering patients, digital tools provide doctors with a comprehensive view of their health through new data streams. These tools are changing the doctor's workflow to provide better care for patients. In 2017, the FDA approved a total of 51 digital health products, reflecting the trend of connectivity and smooth monitoring. These products include a sensor that can be embedded in schizophrenia drugs, allowing patients to share treatment data with doctors through medical apps.
Digital health can also really improve medical outcomes, improve efficacy, and reduce costs. Taking medical imaging software that supports clinical decision making as an example, the diagnosis of stroke requires race against time, as thrombosis can lead to severe loss of function and increase the risk of stroke. In February of this year, the FDA approved a clinical decision support software that uses artificial intelligence algorithms to notify neurovascular specialists at a faster rate, thereby reducing diagnostic time and early effective treatment to save patients.
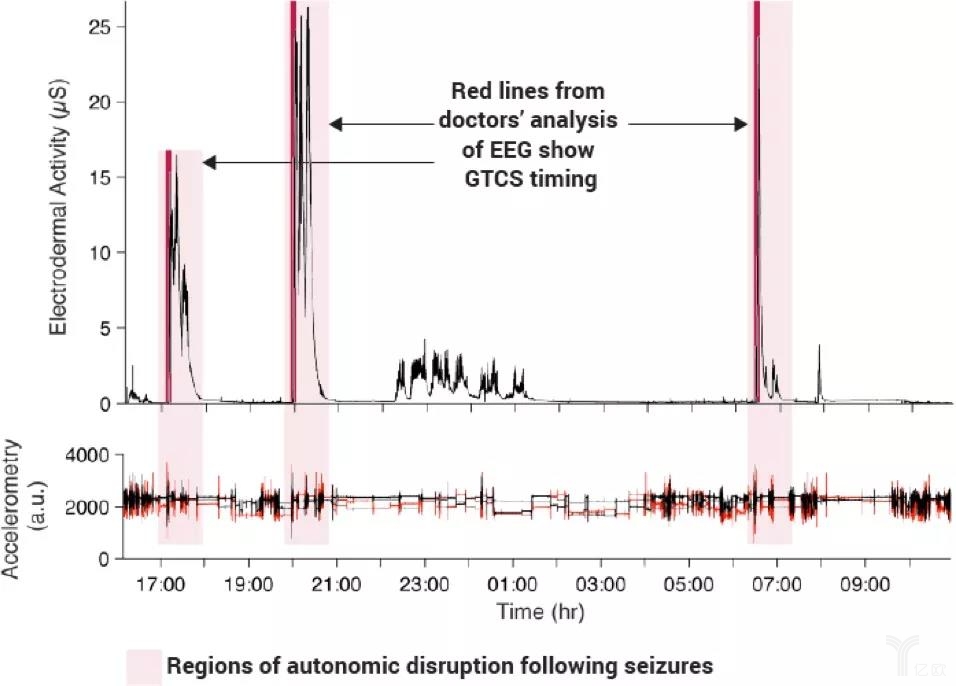
(Approved software uses artificial intelligence algorithms to analyze the timing of seizures, image source: Empatica)
The FDA has played a key role in supporting this continuous innovation as part of our mission to protect and promote public health. First, we must ensure that our regulatory approach maintains our scientific gold standard for testing product safety. We must always put the protection of patients at the top of their work.
As part of our mission, we must also take steps to ensure that beneficial new technologies are effectively promoted and that patients are served in a timely manner. Because ultimately, patients benefit from science.
This means that we must also recognize that FDA's regulatory approach is not always suitable for emerging technologies such as digital healthcare or rapid changes in this area. If we want patients to benefit from innovation, the FDA itself must be as flexible and innovative as the technology we are supervising.
This requires us to adopt modern and flexible regulatory methods in highly innovative areas such as digital health, and encourage more developers to turn advanced technologies into clinical tools to benefit patients. To help drive these opportunities, I announced the Digital Health Innovation Action Plan last summer. This new program outlines our efforts to redesign the FDA's approach to ensure that everyone has access to quality, safe and effective digital health products in a timely manner.
I am committed to using digital healthcare as a tool to empower consumers and break down barriers to providing medical care. In order to expand this progress, I have set several key goals. These include increasing the number and expertise of FDA digital healthcare professionals, launching a digital health software pre-validation pilot project within CDRH, and releasing new guidelines to modernize our policies and outline our efforts to drive digital health innovation.
Digital Health Innovation Action Plan
I am happy to announce that we have made substantial progress on these goals. In addition to what we have achieved, I would like to announce several new initiatives that were first launched.
We have released a new policy that will simplify the path of digital health products with multiple functions, some of which are regulated by the FDA as medical devices. This new guide is another part of our action plan. It explains the FDA's regulatory approach and policies for these versatile digital devices and states that the FDA will or will not review certain software included in these devices as long as they do not pose a risk or disadvantage to the functionality under review by the FDA. influences.
For example, for hospital monitors that detect important information such as heart rhythm and transmit it to the patient's electronic health record, the FDA will only review cardiac monitoring functions unless the transmission function adversely affects the safety or effectiveness of the monitoring function. In this case, developers only need to prove that they have resolved any potential adverse effects between the two different functions.
Our goal is to enable developers to effectively incorporate the latest technologies into their products while focusing FDA review on the safety and effectiveness of high-risk medical devices for diagnosis or treatment. We believe this approach will encourage more innovation in this important area.
I am happy to announce another important update to the program. We have already got a first draft of the working model, which provides a vision for all aspects of the program and steps to expand the plan. This model has been released on our website. We also shared a new roadmap outlining how we will develop the program.
This is the first advanced draft of our iterations of the working model of this new plan. The key part of what we need to advance is the opinions of developers, patients, doctors and the public. So, throughout the working model, you will see that we have developed a “questioning question†about the answers and comments that are needed in each part of the program. The Pre-Cert project we designed is an iterative collaborative experience, and your feedback is the key to its success.
We are committed to launching "Pre Cert 1.0" by the end of 2018, the first version of the program. Once we have firmly mastered this framework, we will further improve it in 2019.
Shampoo,Dry Shampoo,Dandruff Shampoo,Head And Shoulders Shampoo
Guangzhou Lingxue Cosmetics Co., Ltd , https://www.gzlxgj188.com
