Aberdeen dermatitis new drug was approved by breakthrough therapy
January 09, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Biopharmaceutical company AbbVie announced today that the US FDA has given its new drug Upadacitinib a breakthrough therapy for atopic dermatitis for adult patients with moderate to severe atopic dermatitis for systemic therapy. The determination is based on the positive results of its Phase 2B clinical trial published by AbbVie in September 2017. The FDA's Breakthrough Therapy Accreditation Program is designed to accelerate the development and review of ongoing drugs or therapies with initial clinical evidence to support their potential to significantly improve one or more clinically important endpoints.

Atopic dermatitis, also known as eczema, is a chronic inflammatory skin disease characterized by skin ulcers, exudation and scarring of tissue fluids, redness of the skin, intense itching, and dry skin. Symptoms may also manifest as a rash or thickening and hardening of the skin. In the United States, atopic dermatitis affects approximately 28 million people and can seriously affect the physical and mental health of patients.
Upadacitinib is an oral small molecule drug developed by AbbVie that selectively inhibits JAK1. JAK1 plays an important role in the pathophysiological process of immune-mediated diseases. AbbVie is conducting upadacitinib's Phase 2 and Phase 3 clinical trials for atopic dermatitis, rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and Crohn's disease. Upadacitinib has not been approved by the regulatory authorities and its safety and effectiveness have not been determined.
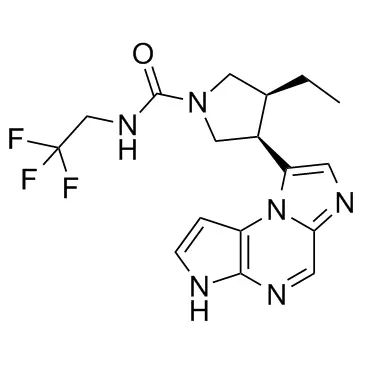
â–²The molecular structure of Upadacitinib (Source: MedChem Express)
Dr. Michael Severino, Chief Scientific Officer and Executive Vice President of R&D at AbbVie, said: "Our R&D history, scientific expertise and leadership in the field of immunization allow us to focus on new therapies for developing urgent and unmet needs. The treatment options for patients with atopic dermatitis are limited, and addressing the needs of these patients is very important to us, and we look forward to advancing upadacitinib to the third phase of atopic dermatitis."
We look forward to more innovative treatments to alleviate the troubles of dermatitis patients.
Reference materials:
[1] AbbVie official website news
[2] Abbvie's Upadacitinib granted breakthrough therapy designation from the FDA for atopic dermatitis
Powder Fire Extinguisher,Powder Type Fire Extinguisher,Dry Powder Fire Extinguisher,Dry Powder Extinguisher
JIANGSU NEW FIRE FIGHTING TECHNOLOGY CO.,LTD , https://www.newayfire.com
