Mary Lame, Hua Yang, Sherri Naughton and Erin Chambers
Waters Corporation (Milford, MA, USA)
Key words
Monoclonal antibody, infliximab, Remicade, protein quantification, eXpress enzymatic hydrolysis
Application advantage
â– Simple, standardized protein quantification method.
â– The universally applicable optimized enzymatic kit eliminates the need for method development steps for new drug development.
■Sample preparation for LC-MS analysis is completed in 4–6 hours.
â– Analysis of infliximab achieved a high sensitivity detection limit of 10 ng/mL.
Waters Solutions
ProteinWorks TM eXpress Enzyme Set Complete Monoclonal Antibody Assay Standard (Part No. 186006552)
ACQUITY UPLC ® Peptide BEH C 18 , 300Å 1.7 μm, 2.1 mm × 150 mm Column for Peptide Analysis (Part No. 186003687)
ACQUITY UPLC
Xevo® TQ-S Mass Spectrometer
ProteinWorks μElution SPE Purification Kit
Introduction
As more and more drug developments focus on large molecules such as antibodies or ADCs, scientists who study traditional "small molecules" face challenges that are not only due to the complex and time-consuming nature of small molecule research, but also from the use Multiple workflows that may be involved in LC-MS for protein quantification. Researchers who frequently use ELISA and other immunoaffinity (IA) methods to study protein biomarkers face the same problem. Although the IA method is very sensitive and simple to operate, its reagent reproducibility is poor, lack of standardized methods and cross-reactivity, limited linear dynamic range, and other shortcomings, which promotes the development of methods for switching to LC-MS, especially In the field of discovery research and early development / preclinical research. However, the LC-MS workflow consists of multiple subsections, each of which contains many steps. The sensitivity of the specific reagents, as well as the time, temperature, and reagent concentration of each step, can make it difficult to quickly obtain a method that achieves the desired detection limit. This application note describes a rapid and sensitive method for the quantitative analysis of infliximab in rat plasma using the ProteinWorks eXpress enzymatic kit and protocol (Figure 1). Analysis of infliximab with a comprehensive set of sample preparation methods using pre-weighed and batch traceable reagents under a series of carefully developed general-purpose, simple step-by-step instructions LLOQ (lower limit of quantitation) at 10 ng/mL.
experiment
sample discription
Infliximab in 35 μL rat plasma was first immunopurified using Protein A agarose-based 96-well plates. Samples were then processed using the ProteinWorks eXpress enzymatic kit and protocol for LC-MS analysis. Finally, the characteristic peptides were purified using the ProteinWorks μElution SPE purification kit and protocol.
Figure 1. Protein structure of infliximab (Remicade) E.
LC condition
LC System: ACQUITY UPLC
Detection: Xevo TQ-S mass spectrometer, ESI+
Column: ACQUITY UPLC ® Peptide BEH C 18 , 300Å 1.7 μm, 2.1 mm × 150 mm Peptide column temperature: 55 °C
Sample temperature: 10 °C
Injection volume: 10 μL
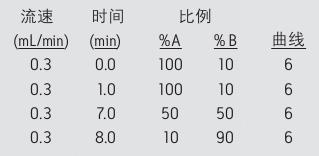
Mobile phase A: 0.1% formic acid aqueous solution mobile phase B: 0.1% formic acid in acetonitrile solution
gradient
MS condition
Capillary voltage (kV): 3
Cone hole voltage (V): 30
Ionization source compensation (V): 50
Ion source temperature (°C): 150
Desolvation gas temperature (°C): 600
Cone gas flow rate (L/h): 150
Collision gas flow rate (mL/min): 0.15
Sprayer airflow (Bar): 7
Data management
MassLynx® (version 4.1)
Results and discussion
As the US patent expiration deadline for infliximab is getting closer to 2017, CROs (contract research institutes) and biosimilar research laboratories are increasingly paying attention to this important drug. However, typical workflows are extremely complex and have many options and options. This makes the development of high sensitivity methods full of challenges. In this application note, we use the ProteinWorks eXpress Enzymatic Kit to streamline and streamline processes. Affinity purification, enzymatic hydrolysis, and peptide extraction of infliximab samples were completed in less than 6 hours using the SPE method. This method allows us to start collecting data on the same day, and there are still more 96-well plates running the next morning. We also monitored a variety of unique characteristic peptides and a universal human peptide for quantification. The best sensitivity was obtained using the characteristic peptide SINSATHYAESVK from the heavy chain, and the monitoring data of other characteristic peptides of infliximab (DILLTQSPAILSVSPGER, light chain) and the universal peptide (DSTYSLSSTLTLSK, light chain) were used for the confirmation of the results. A characteristic peptide (SVSELPIMHQDWLNGK) from the normal mouse mAb standard (part number 186006552) was used as an internal standard.
Using the optimized protocol and reagents provided in the kit, only 35 μL of plasma can achieve a detection limit of 10 ng/mL in the infliximab assay (Figure 2). Table 2 summarizes the linearity and accuracy of the standard curve for each peptide. The main quantitative peptide, SINSATHYAESVK, has the highest sensitivity and remains linear over a range of more than 4 orders of magnitude, with an average accuracy of more than 98% for all points on the standard curve. The other two peptides remain linear over a range of more than 3.5 orders of magnitude, and the average accuracy of all points on the standard curve is greater than 99%.
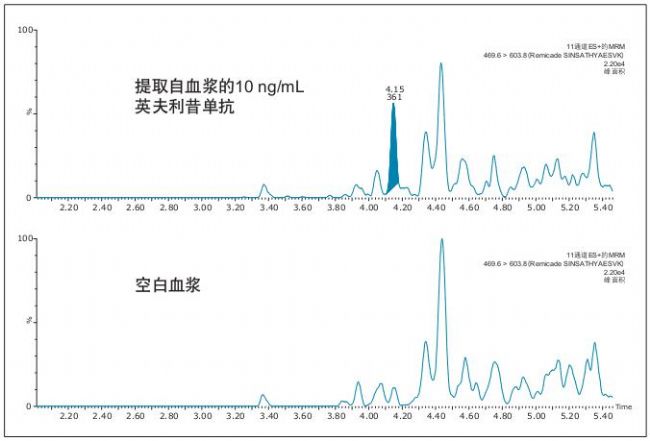
Figure 2. Chromatogram of rat plasma samples showing infliximab at a concentration of 10 ng/mL (compared to blank rat plasma samples). Infliximab was quantified using the characteristic peptide SINSATHYAESVK.
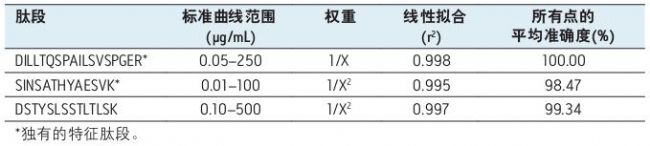
Table 2. Linear dynamic range and standard curve statistics for the characteristic peptides used to quantify infliximab in rat plasma.
In addition, QC samples are excellent in accuracy and precision, with all coefficient of variation (%CV) less than 6%. The relevant data is summarized in Table 3. In fact, the QC sample of the SINSATHYAESVK peptide has an average coefficient of variation of less than 3%.
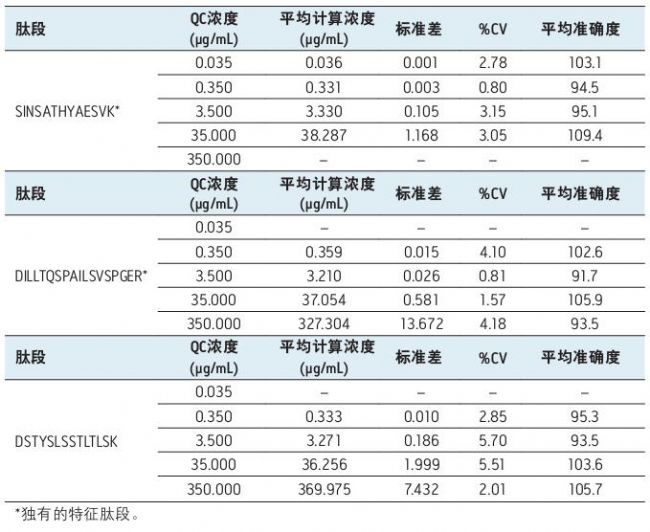
Table 3. Statistics of all infliximab peptide QC samples used for quantification.
It is evident from the chromatographic data evaluation that the data quality improvement in peak width and separation of residual endogenous components has enabled us to achieve low concentration levels and achieve high accuracy and precision. This conclusion can be drawn from the QC chromatograms of all of the characteristic peptides in Figures 3-5 (and highlighted in the figure).
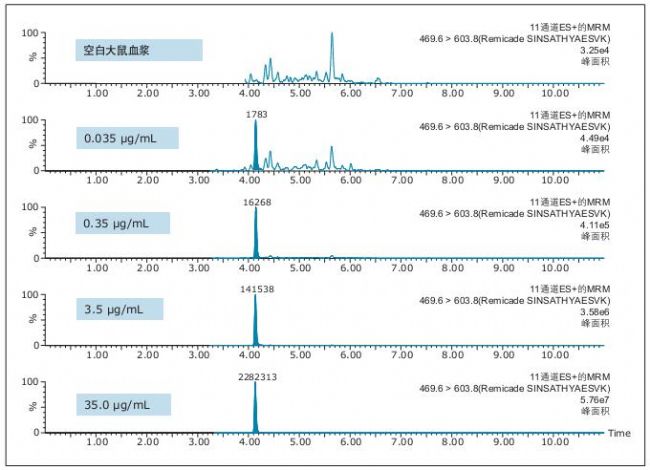
Figure 3. QC chromatogram of the unique characteristic peptide SINSATHY AESVK of infliximab.
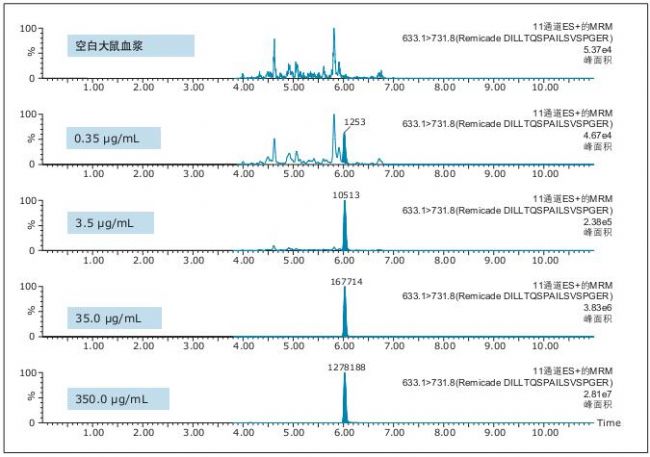
Figure 4. QC chromatogram of the characteristic peptide DILLTQSPAILSVSPGER unique to infliximab.
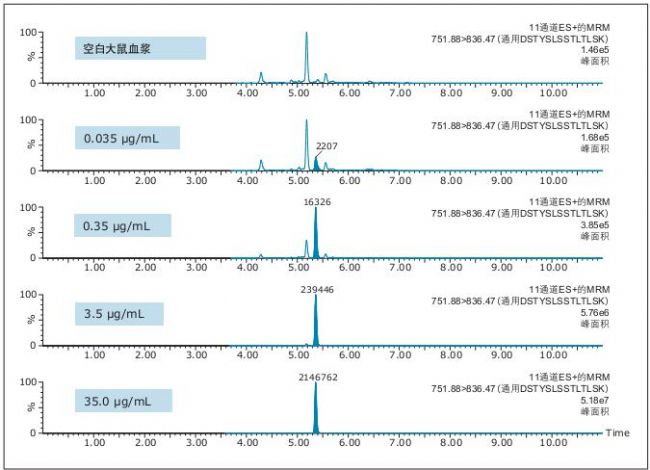
Figure 5. QC chromatogram of the general characteristic peptide DSTYSLSSTLTLSK of infliximab.
in conclusion
We successfully purified infliximab using a series of typical standard curves and rat plasma QC samples using the ProteinWorks eXpress Enzyme Set. The quantitative limit of 10 ng/mL was easily achieved while maintaining excellent linearity and single digit accuracy. The total sample preparation time including the affinity purification step is no more than 6 hours. The total enzymatic preparation time is only 2 hours. The kit-based general approach allows beginners to achieve ultra-low detection limits with a simple step-by-step protocol and a range of standardized, pre-tested reagents, ensuring the required sensitivity while ensuring flexibility Conversion.
references
1. McKinsey and Company; Data Source: Evaluate Pharma, US Patent Expiration Dates.
Waters China Ltd. Waters Technology (Shanghai) Co., Ltd. Beijing: 010 - 5209 3866
Shanghai: 021 - 6156 2666
Guangzhou: 020 - 2829 5999
Chengdu: 028 - 6765 3588
Hong Kong: 852 - 2964 1800
Free after-sales service hotline: 800 (400) 820 2676
Bok Choy,Chinese Cabbage,Green Vegetables,Dried Green Vegetables,Dehydrated Green Food
Jiangsu Tiankang Food Co., Ltd. , https://www.tiankangfood.com
