Recently, cases of using chemical raw materials to make " poisonous yuba " have appeared in many places in the country . Hanging white blocks, borax, and urotropine are all toxic and hazardous substances that are prohibited from being added to food. The criminals use these chemical materials to increase the weight, bleach, anti-corrosion and toughness of yuba to gain illegal benefits.
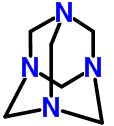
The chemical name of urotropine is hexamethylenetetramine, which is an industrial raw material, which can whiten and preserve freshness, increase the taste and antiseptic effect. Ingestion can lead to allergies, carcinogenesis, teratogenic and other hazards. In 2010, China was listed as the fourth batch of "List of non-food additives that may be illegally added in food", which is banned from being used in food production.
Liquid quality analysis method for urotropine in liquid chromatography-mass spectrometry/mass spectrometry based on the standard "SN/T 2226-2008 for the detection of urotropine residues in animal foods imported and exported", Waters users Special pretreatment methods have been developed for urotropine in soy products. The method uses an MCX cartridge with dual functions of reverse phase and ion exchange to maximize the retention of the target and to remove plant protein interferences from the extract to obtain the cleanest sample. The HILIC mode column enhances the retention and sensitivity of the compound in the acidic mobile phase.
Test method (applicable to yuba, bean skin and other soy products and rice noodles):
extract:
Accurately weigh 1.0 g sample, place it in a 50 mL plug colorimetric tube, add 5 mL of 80 ° C hot water, shake and let stand for 10 min.
The volume was adjusted to 25 mL with 1.5% trichloroacetic acid aqueous solution, and homogenized and ultrasonically extracted for 5 min.
The extract was transferred to a 50 mL centrifuge tube, centrifuged at 10,000 r/min for 10 min (temperature below 15 °C), and 5 mL of the supernatant was taken for purification.
Purification: Oasis MCX cartridge (3CC/60mg, Part No.186000253)
Activation, equilibration: 3 mL methanol, 3 mL water sample: 5 mL extract wash: 3 mL water, 3 mL methanol elution: 3 mL 5% ammoniated methanol.
After drying under nitrogen, the residue was dissolved in 1 mL of 0.1% acetic acid/acetonitrile (2+8) and passed through a 0.22 μm filter.
LC/MS/MS analytical method (SN/T 2226-2008)
| Column: | UPLC column: ACQUITY BEH HILIC 2.1*50 mm 1.7 μm (Part No.186003460)* |
| Mobile phase: | 0.1% acetic acid / acetonitrile (2/8) |
| Flow rate: | 0.25 mL/min |
| Injection volume: | 5 μL |
| Column temperature: | 30 °C |
| Mass spectrometry: | Positive ion, multiple reaction monitoring mode parent ion 141.1 Child ion 112.2/98.2 |
* HPLC method can use XBridge HILIC 2.1*50 mm 3.5 μm (Part No.186004433) HPLC column, and other experimental conditions can be adjusted accordingly.
Chickenpox Vaccines,Varicella Virus Vaccine Live,Varicella Vaccine Single Dose Vial,Chickenpox Vaccines Vial
Changchun BCHT Biotechnology Co. , https://www.ccbcht.net
