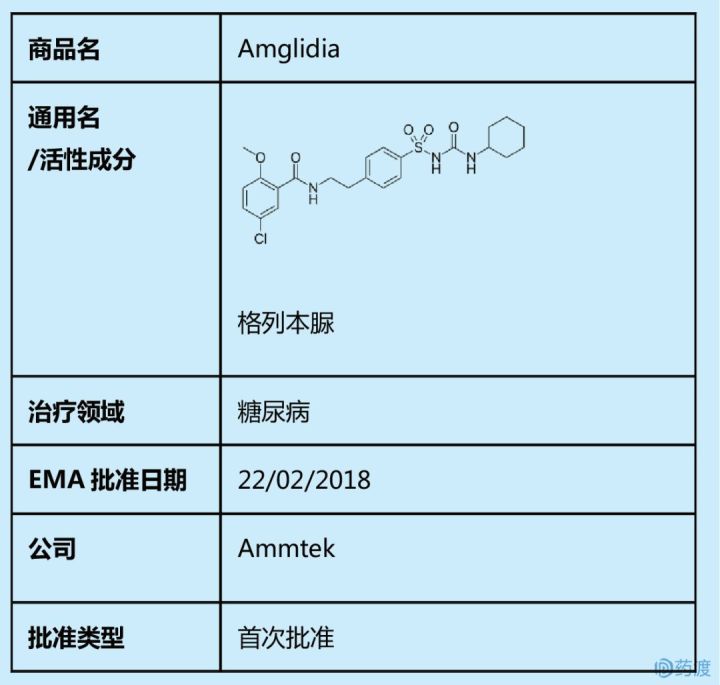
The first oral neonatal diabetes drug was approved by the EMA
February 24, 2018 Source: Yaodutoutou
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];The European Medicines Agency (EMA) Human Drugs Committee (CHMP) has recommended that the European Union grant Ammtek's Amglidia marketing approval, the dosage form is oral suspension, specifications 0.6mg/mL and 6mg/mL, becoming the first approved treatment Oral medication for neonatal diabetes.

The drug was awarded the orphan drug title on January 15, 2016 and was first approved by EMA for the treatment of neonatal diabetes (NDM) on February 22, 2018. Amglidia active ingredient glibenclamide belongs to the class of sulfonylurea hypoglycemic agents, which stimulates islet β cells to secrete insulin to control blood glucose by blocking ATP-sensitive potassium channels, and transient newborns encoding KATP channel gene mutations and chromosome 6q24 abnormalities. Diabetic patients are effective, and their most common side effects are hypoglycemia, transient diarrhea and abdominal pain.
Dalenil was first marketed in Europe in January 1969. The Amgindia approved indications and dosage forms were different. The study showed that Amglidia has good efficacy and bioavailability for the treatment of NDM.

Neonatal Diabetes (NDM) is an extremely rare form of diabetes with hyperglycemia occurring within six months of postpartum, at least 2 weeks, with a risk of ketoacidosis. According to different clinical symptoms, NDM is divided into temporary neonatal diabetes and permanent neonatal diabetes. The former can relieve or disappear spontaneously within 3-6 months of onset, but about half will relapse in adolescence; the latter will continue. Onset. The pathogenesis of NDM is unknown, and mutations in different genes that cause this type of diabetes have been discovered.
Currently, methods for treating neonatal diabetes are usually in which the caregiver uses insulin for the patient according to the prescription or parent at home, or super-labeled for the adult-only glibenclamide that can be purchased. In order to make the drug suitable for newborn and pediatric patients, the tablets are usually crushed and mixed with a small amount of water, and then the oral syringe is used to assist the patient. This mode of administration may result in a low or overdose medication risk. Amglidia makes glibenclamide more accurate to meet clear unmet medical needs. In addition, patients treated with Amglidia may not require insulin therapy or only low doses.
Amglidia has benefited clinically from data published in the literature as well as data from bioavailability studies and NEOGLI clinical studies. Because the disease is very rare, only 10 patients participated in the NEOGLI clinical study, and the results showed that glycemic control remained stable after conversion from crushed tablets to oral suspension.
Since NDM was a very rare disease, Amglidia was awarded the title of Orphan in January 2016. At the time of approval, the drug's orphan drug title will be reviewed by the EMA's Orphan Drugs Committee (COMP) to determine whether the current information can maintain Amglidia's orphan drug status and grant the drug 10 years of market exclusivity.
Reference: EMA News
China Extract Powder For Use As Dietary Supplement Extract Powder, Extract Powder Manufacturer
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.bio-pharmacies.com
