1 Preparation of bacterial solution
1.1. Take the glycerol solution containing the target plasmid (-80 ° C or -20 ° C in the refrigerator), after the bacterial solution is melted, use the inoculation loop on the ultra-clean table to streak the ampicillin-resistant plate, and incubate at 37 ° C. -16h. When the plate grows colonies, select monoclonal colonies that are in good growth. If there is no bacterial solution of the target plasmid, it is necessary to take the stock plasmid for transformation, and then pick the monoclonal shake.
1.2. The monoclonal was inoculated into 5 ml of ampicillin-resistant LB liquid medium, and cultured at 37 ° C, 220 rpm / min for 12-16 h;
1.3. Dispense the above 5 ml LB broth into 5000 ml of 2 2 ml centrifuge tubes for 8 min, discard the supernatant (pour the tube onto the paper towel for a few minutes, remove the supernatant), and set the bacteria for use.
2 Extraction of adenovirus plasmid (refer to AxyPrepTM Plasmid Mini Kit)
2.1 Take 250ul Buffer S1 that has been added to RNase A to suspend the bacterial pellet. The bacterial pellet should be blown evenly, and no small bacteria should be left.
2.2 Take 250ul Buffer S2 into the above-mentioned bacterial suspension, gently and fully flip up and down 6-8 times to mix well to fully lyse the bacteria until a clear solution is formed;
2.3 250 ul 4 ° C pre-cooled Buffer S3 was added to the above solution, gently and fully flipped up and down 10 times to mix until a compact agglomerate was formed and allowed to stand at room temperature for 5 min.
2.4 Transfer the supernatant to a small extraction tube, centrifuge at 5000g for 1min at 4°C, then remove the small extraction tube from the 2 ml centrifuge tube and discard the filtrate from the centrifuge tube.
2.5 Place the preparation tube back into the centrifuge tube, add 0.5 ml Buffer W1,5000g for 1 min, and discard the filtrate.
2.6 Place the preparation tube back into the centrifuge tube, add 0.7ml Buffer W2, centrifuge at 5000g for 1 min, and discard the filtrate; in the same way, operate again with 0.7ml Buffer W2 and discard the filtrate.
2.7 Finally, place the small extraction tube back into a 2 ml centrifuge tube, centrifuge at 12,000 g for 1 min, and dry the alcohol.
2.8 Then take out the small extraction tube, place it in a clean 1.5 ml centrifuge tube (provided in the kit), add 60-80 ul of Eluent to the membrane of the small extraction tube, let stand for 1 min at room temperature; then centrifuge at 12000 g After 1 min, the centrifugation solution was collected, which was a small sample of the initial solution and stored at -20 °C.
2.9 Take appropriate amount of plasmid initial solution for plasmid concentration determination for subsequent plasmid linearization test.
3 Enzymatic cleavage linearization of adenovirus plasmid
The small plasmid was linearized by PacI and the system was as follows:
10×NEB Buffer I | 10 ul |
Plasmid | 10-20ug |
100×BSA | 2.0 ul |
Pac I (15 U/ul) | 1.0 ul |
Add ddH2O to | 200.0 ul |
The enzyme was cut overnight (about 17-20 h) in a 37 ° C incubator.
4 Linearized adenoviral plasmid purification
4.1 The above overnight digested product was briefly centrifuged and transferred to a sterilized inlet EP tube; then 200 ul (equal volume) of chloroform was added, mixed upside down, and centrifuged at 12000 rpm for 10 min.
4.2 Carefully pipe the upper liquid into a new sterilized inlet 1.5 ml EP tube and add 1/10 volume (supernatant volume) of NaAc (3M pH 5.2) and 2.5 volumes of absolute ethanol (-20 ° C pre-cooled). Mix upside down, and the obvious floc will be DNA. Place it at -20 °C for 2-3 h and centrifuge at 13,000 rpm for 15 min.
4.3 Discard the supernatant, add 1ml of 75% ethanol, finger the bottom of the EP tube several times, resuspend the pellet, centrifuge at 8000 rpm for 5 min, discard the supernatant; repeat this step.
4.4 Repeat step 3.4.3, soak the pellet for 10min, remove the centrifuge tube after centrifugation, take the centrifuge tube to the cell biosafety cabinet, and discard the supernatant as much as possible (preferably low pressure pumping, so fast and clean) You can also use 10ul gun to suck the residual liquid to prevent the sediment from being sucked away. Open the lid in the safety cabinet to dry the alcohol, and the precipitate can be changed from white to transparent.
4.5 Add 10-20ul sterile ddH2O to dissolve the precipitate, dissolve at 4 °C overnight, flick / evenly with a gun, remove 0.5 ul and add 2ul ddH2O (5 times dilution), then determine the DNA concentration by electrophoresis for concentration determination. The plasmid was stored at -20 °C.
4.6 Calculate the plasmid concentration, record and mark it, and store at -20 °C.
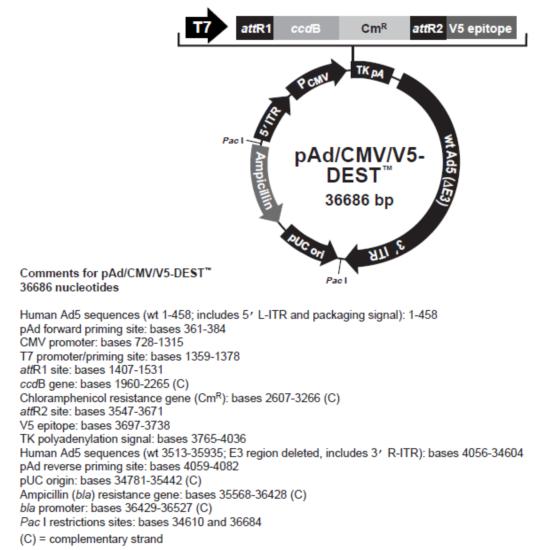
Adenovirus expression vector map:
Continuous release...
Reference link: http://
China Funeral Body Bag,Corpse Body Bags manufacturers, welcome Morturary Body Bags,Funeral Dead Body Bag purchasers from worldwide to visit our site.
Funeral Body Bag,Corpse Body Bags,Morturary Body Bags,Funeral Dead Body Bag
Medton Medical , https://www.medtonmedical.com
