Chongqing Fuchuang Pharmaceutical Research Co., Ltd. ("Fuchuang Medicine") was established in 2009 by a team of overseas scientists and Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharmaceutical") and its member companies. A national high-tech enterprise that researches and develops small molecule chemical drugs.
In the nine years since the establishment of Fuyin Pharmaceutical, more than ten innovative small-molecule innovative drugs with independent intellectual property rights have been developed, and five products have been approved by clinical trials. Among them, FCN-159, which is mainly used for the treatment of advanced solid tumors, has just passed the approval of the clinical trial of the State Food and Drug Administration.
Recently, Fuchuang Pharmaceuticals, which has just moved to the Chongqing Science and Technology Research Institute, has ushered in a new milestone. “With the rapid development of the company, the original site can no longer meet the needs of research and development. It is imperative to build a new site. In order to better adapt to the rapid development of the company, to provide more powerful support for the focus on innovative research and development, Fuchuang Pharmaceutical will be relocated this year. To Chongqing Science and Technology Research Institute.†Wang Weibo, President of Fuchuang Medicine, introduced.
As a chemical innovation platform for Fosun Pharma, Fuchuang Pharmaceutical is based in Chongqing and is committed to innovation and research and development. At present, Fuchuang Pharmaceutical's international layout of “three countries and three places†in Chongqing, Shanghai and San Francisco has achieved a 24-hour continuous research and development model to rapidly promote the best small molecules for cancer and metabolic diseases. Chemical drugs enter the clinical research stage.
The new site of Fuxin Medical has a total area of ​​nearly 2,000 square meters, including chemical laboratories, biological laboratories and offices, equipped with more advanced and complete instruments and equipment. The chemical laboratory is equipped with advanced and complete synthetic and analytical instruments. The biological laboratory has certified SPF (Specific Pathogen Free) animal laboratory, 10,000 clean cell laboratory and molecular biology. The laboratory provides strong support for innovative drug development.
In the new address, Fosun International Co-President, Fosun Pharma Chairman Chen Qiyu and Fu Chuang Pharmaceutical President Wang Weibo answered questions on media concerns. The following is the text:
Q1: Please introduce the international layout, commercialization ideas, plans and strategies of Fuxin Pharmaceutical in Chongqing, Shanghai and San Francisco.
Wang Weibo: Re-creation is a small molecule innovative drug research and development platform under Fosun Pharma. It focuses on the discovery stage in the early stage and is in the clinical and commercial stage in the later stage. It also hopes to promote the development of Chongqing biomedical industry and attract more talents through rehabilitation. Come in and make the industry bigger and stronger, so from the first ten years to the present, we are ready to slowly put the products in Chongqing.
Regarding the layout of the three countries and the two places, Chongqing is our home base and the main battlefield where we are rooted. On the one hand, there are many talents here, and there are many talents in universities such as major, heavy medical, Huaxi, and Sichuan University. After several years of entrepreneurship, Chongqing's team is very united, sincere and dedicated. We appreciate this quality and have great advantages compared with the coastal areas, which can attract talents from the southwest.
San Francisco will lay the cutting edge of cutting-edge technology, whether it is biotechnology or structure-based design, we use the latest information and technology in the United States to layout, especially in international multi-center clinical, clinical aspects will also lay out some high-end CMO or R & D personnel In San Francisco.
As for Shanghai, because Fosun Pharma is headquartered in Shanghai, Shanghai has many CROs, so we will also lay out. Fosun is using China Power to graft global resources, and the same is true for reinventing, developing new drugs with the most effective resources, the fastest speed and the lowest cost.
Chen Qiyu: In the past, the team size of Fuchuang Medicine was not very large. It focused on the front-end R&D. It is indeed a business model that it is more important to consider candidate compounds before starting to find partners.
But now with the improvement of Chinese regulations, innovative pharmaceutical companies can not do their own production. Marketing itself has a very mature model to cooperate, so from the perspective of reinvention, after accumulating so long experience in innovative pharmaceutical development, combined with China's policy environment and market environment, the state also encourages innovative medicine. China quickly approves the listing, and the capital market also welcomes such a model. Reinventing the trend is to upgrade the strategy. It will only find partners after a certain period of time. The partners will bear more future investment and risks and share the benefits with the reinventing. The mode is changed to a more complete closed-loop mode, and the whole process is promoted by itself.
I believe that such a model will be more efficient, because one thing from start to finish, a product from research and development to approval to go public, the entire link in addition to the manufacturing process to the partners to do, the other links are in their hands, this It is the most efficient, is conducive to the overall resources, is conducive to efficient cooperation with clinical institutions, and is also conducive to further deep cooperation with many partners around the world.
This strategy is good for Fuxin and Fosun. In the past, some of the links were transferred by Fosun Pharma's own company. Of course, it will not be ruled out in the future, but from the perspective of rehabilitation, Some products can be opened to maximize value.
Q2: Since 2002, Fosun Pharma has been investing in Chongqing, especially in the research and development of pharmaceuticals, including Pharmacy Pharmaceuticals, Rehabilitation Medicine, Chongqing Medical Engineering Institute, etc., which have achieved innovation and internationalization. Excellent results, why is Chongqing the center of research and development in the west?
Chen Qiyu: Chongqing itself has a very good foundation. Fosun Pharma initially entered Chongqing investment, which was in 2002.
In the past two years, Yaoyou Pharmaceutical has also had a very deep layout in R&D investment and team building. In terms of consistency evaluation, Yaoyou Pharmaceutical has done a lot of work, and it is the first in Chongqing. There will be a lot of work to be promoted. In the past, Yaoyou Pharmaceutical was specialized in liver disease, antibiotics, etc. Recently, it has completed the strategic integration of Hunan Dongting Pharmaceutical Co., Ltd., and is a psychotropic drug.
Fosun's pharmaceutical industry layout in Chongqing is to meet international standards. Whether it is the research and development and manufacture of API intermediates, Kailin Pharmaceutical, a subsidiary of Yaoyou Pharmaceutical, must meet global standards and supply to the world. The generic drug research and development capabilities are evaluated from the consistency, including the drug users themselves are also certified by the FDA system. There are also innovative drug research and development, and the model of the two countries guarantees that the main work of the product is produced in Chongqing, but it also meets the globalization standard.
Therefore, the founding base camp is still in Chongqing, especially after the completion of the new site today, the carrying capacity and level of the entire platform have been greatly improved. Building a complete pharmaceutical innovation industry chain and industrialized industrial chain in Chongqing are mature. Here we have hundreds of innovative R&D teams, which will be further strengthened in the future, making Chongqing a high-efficiency and high-level The innovation center of the full value chain. In the future, it will be inevitable that several different companies of Fosun will aggregate and form an industrial chain in Chongqing to create an international three-dimensional industrial system capable of meeting global standards.
Q3: In September, the anticancer drug FCN-159 was accepted and accepted by the clinical trial registration of the Food and Drug Administration. After the Fosun Pharma industry and the rejuvenation medical conditions were met, the clinical phase I trial of the new drug was carried out. What is the current progress and product line?
Wang Weibo: Why is it called FCN-159? F is the meaning of invention. CN is China and represents China. Cancer should be combined with drugs. In this aspect, Fosun has a strong advantage. Rehabilitation can be used in combination with drugs. In the future, we will treat some refractory rectal cancers. Now we are laying out. This is the case of FCN-159.
Regarding the current product line layout, our main purpose is to meet some unmet needs in the field of small molecule therapy. Now it seems that cancer is the biggest demand. It turns out that patients have cancer without expecting it. Now through scientific development, cancer It has gradually become a chronic disease, so our entire layout still uses cancer as the main direction.
The other is Alzheimer's disease, because people's life expectancy is getting longer and longer, so the demand is very large. In the case of qualification and ability, it is still necessary to enter the difficult and conquered, original, and truly meet the needs of patients, so we are committed to overcoming some difficult areas, and truly rival the international companies in originality.
In strategy, Recycling will quickly follow up on existing targets, improve the numerator, and make better products than other companies.
The target of diabetes is now being done, and we are closely following and actively tracking. However, diabetes has a problem, especially in the United States, which is very toxic to the heart. It requires a large-scale clinical trial, and the investment is very high and the risk is very high. We must now consider what kind of volume we have and what kind of risks we bear, so the overall layout is such a reflection.
Q4: The pharmaceutical industry is one of the pillar industries of Chongqing Liangjiang New District. As a chemical innovation platform for Fosun Pharma, Fuchuang Medicine is based in Chongqing and is located in the Industrial Park of Liangjiang New Area. The future functions and positioning include the main direction. What is the output value and so on?
Wang Weibo: Regarding the location of the site, we are very honored to be located in the treasure of the Institute of Science and Technology of Liangjiang New District. It is indeed very suitable for scientific research. At present, the area is 2,000 square meters. We have a chemical area of ​​nearly 600 square meters. It should be world-class. There is also a supply optimization room downstairs, and the most advanced biological screening center in Chongqing. It is divided into three parts: molecular biology experiment. Room, cell laboratory and animal laboratory.
The cell laboratory is close to 10,000, and the standard is very high. There are more than 50 cancer cells that are difficult to obtain. These are all made by ourselves, and some are bought from international suppliers. Animal laboratories can also be said to be the best in Chongqing. From the perspective of the overall animal model, in terms of cell-level technology, we are in line with international standards and are world-class.
More than 90% of the students are master students. We hope to slowly gather some talents in biomedicine and build a better platform, including the introduction of R&D teams and institutions from overseas. Every year, the staff and research team will work in San Francisco for a period of time. This process is also to align the local talent level with internationalization.
Drug research and development requires the wisdom of many people. We still have to do clinical research, do international business cooperation, and need international talents to come to Chongqing. Not only that, in technology such as the use of artificial intelligence to design drugs, our core technology advantage is based on structural drug design, but also computer simulation of proteins and small molecules, in some sense, artificial intelligence algorithms. We are also actively cooperating with foreign countries. This is definitely a layout.
Chen Qiyu: There are more than 4 million new-onset patients with oncology drugs in China. The clinically innovative oncology drugs are generally priced from tens of thousands to 200,000 yuan, because they are now more precise targeted therapies. For example, non-small cell lung cancer is relatively accurate, so it will be targeted at precise people when it goes on the market, and it is aimed at products with certain market demand.
In principle, every drug comes out with at least several hundred million yuan of sales. This is relatively conservative. Generally, innovative tumor drugs are in the Chinese market. Multinational companies can now sell products worth between 2 billion and 3 billion yuan. .
The energy of the future products will be so large in China. At the same time, because we are independent research and development, we have global intellectual property rights. Generally speaking, China currently only accounts for 5%-10% of the global market share of innovative drugs. In other words, the world The market opportunity is 10-20 times as large as the Chinese market. In the future, we will have to expand the ability of output through various means. Some of them are jointly developed with the permission of multinational companies, and some of them advance forward and cooperate with various modes.
Q5: The bio-pharmaceutical industry has become one of the areas with good growth and active development in the modern industrial system. In the medical field, the country has a lot of policies. Is it now the golden age of biomedicine? If so, how long should it last?
Chen Qiyu: Chinese pharmaceutical companies have entered a period of very good development of innovative drugs, biomedicine, including small molecules, and highly difficult drugs. This period lasts at least 20 years.
One is the cycle of the drug, 8-10 years of research and development, when the product comes out, because of patent protection, at least 10-15 years, so this wave of Chinese pharmaceutical companies in the innovative drugs, will generate the cost of Chinese companies The two advantages of the market, the superposition effect.
We have such a huge market, there are more than 4 million patients with tumors. In such a large market, if Chinese companies can cross the past with R&D, we will meet the needs of the Chinese market with high cost performance. Based on this, we also It can deliver high-performance, affordable and innovative medicines to the global market.
From this point of view, when we continue to develop a large number of innovative drugs in the future, the Chinese pharmaceutical industry will have a major impact on the global pharmaceutical innovation chain. The cost structure, market factors and models of global drug innovation will occur very much. Big changes, including China's capital markets, will also greatly support innovative drugs.
In the future, when these drugs are on the market, a decade or so is a very golden stage for these drugs. So, for at least 20 years, because innovative drugs are relatively long-cycle industries, even 30-40 years.
Q6: Re-creation is the only small-innovative pharmaceutical company in Chongqing. Since its establishment 9 years ago, Fuchuang Pharmaceutical has conducted research and development of more than ten targeted innovative drugs with independent intellectual property rights. Five products have been approved by clinical trials. There are many companies that innovate medicines. What are the advantages of reinventing competition?
Chen Qiyu: Innovation still has first-class talents. This is a foundation. In particular, the innovation of medicine, because it is ultimately used on people, but also to solve problems, but also to minimize risks, this is a very complicated science. Innovative drugs are competing with global pharmaceutical companies. Whether new drugs can be approved, the evaluation criteria for effectiveness and safety are internationalized and global, so first-class talents are needed.
In addition, the team must be able to withstand loneliness. It is time to recreate it. Today, we have only 5 products in the first phase of clinical trials. There are still many ways to go in the future, so we must live.
The third is to have the right investors. If the investors themselves insist on it, what is his starting point? If it is only from the perspective of capital profit-seeking, the investor structure is very important. For Fuxing, Fosun Pharma is not just a simple investor. Re-creation is part of our strategy and business, so we can persist. No matter how the capital market and the external environment fluctuate, we must insist on this. Go on.
Poleline Hardwares
Poleline Hardware or Power line accessories / fitting widely used in connecting, handling and holding various overhead line systems, insulators, wires, and other utilities. It is an important component of the pole line construction. According to the application, it can be divided into Telecommunication pole hardware, Utility pole mounting hardware Transmission and Tower hardware.
Yokelink supply a full line of Poleline Hardware, we offer from the top of the pole to underground.
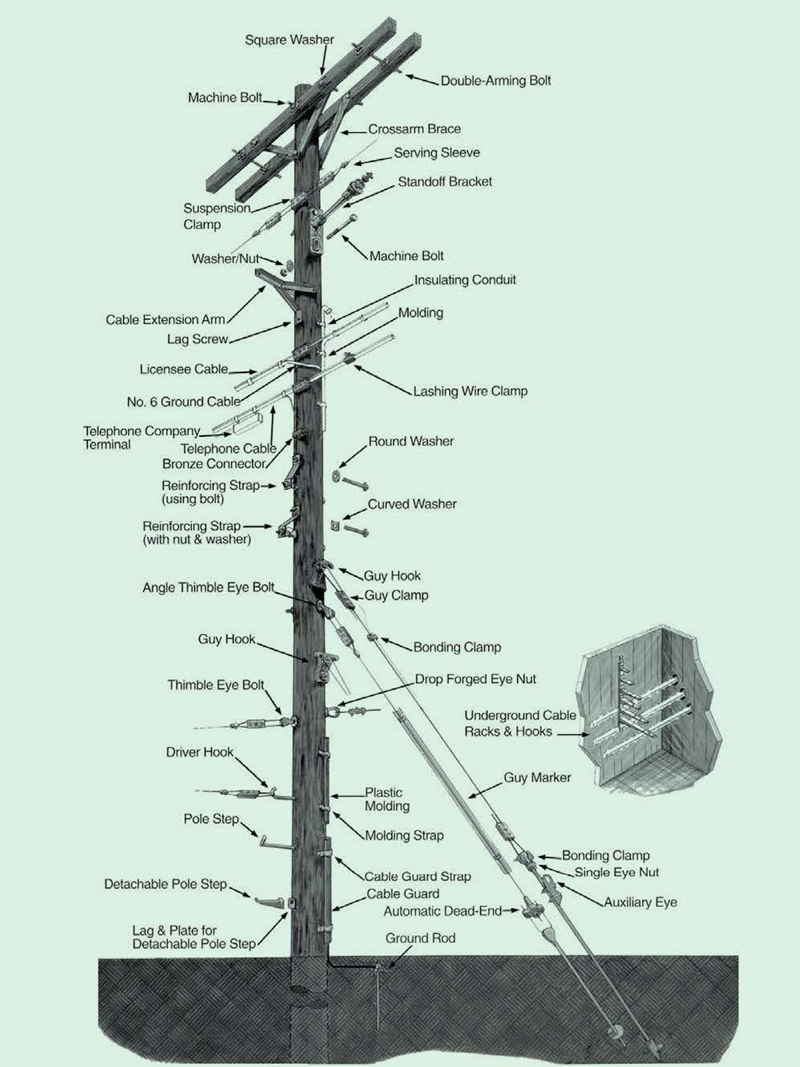
Pole Line Hardware,Poleline Hardware,Transmission Hardware,Telecommunication Hardware
Ningbo Yokelink Machinery Co.,Limited , https://www.yokelink.com
