Medical Network June 21st, recently, the song ceremony announced on the official website, the company's first domestic research and development of the first native hepatitis C class 1 new drug danovir sodium tablets (also known as Danolive sodium tablets, trade name Gono Wei) Approved by the State Drug Administration. Since 2017, six hepatitis C drugs have been approved for listing in five pharmaceutical companies in China. Except for songs, the rest are multinational companies. In addition, in the list of drugs that have been included in the priority review, in addition to the approved listings, there are 18 acceptance numbers (involving 11 varieties) for hepatitis C drugs, of which 3 are imported varieties.
Black Horse Songs "Outline"
The first domestic new hepatitis C drug listed
On June 13th, the song ceremony announced that its self-developed hepatitis C class 1 new drug danovir sodium tablets (also known as Danolive sodium tablets, trade name Gonowei) has been approved by the State Food and Drug Administration for listing. MED Drug Evaluation Database 2.0 data, the gift of Danolivir sodium tablets has been in the process of approval - pending certification.
Figure 1: Review and approval of Danonevir sodium tablets

(Source: Minenet MED Drug Review Database 2.0)
Gonowei is the first product to be approved for marketing, and it is also the first domestically developed self-developed hepatitis C DAA (direct anti-viral) drug. On January 3, 2017, Gonowo's listing application was accepted by CDE, the acceptance number was CXHS1600012, and on October 30, 2017, the acceptance number entered the three-in-one review sequence, June 11, 2018, State Food and Drug Administration After the approval was completed and the production was approved, it took 524 days from the time of application for listing to the approval of the listing.
Figure 2: Danovire sodium tablets review timeline
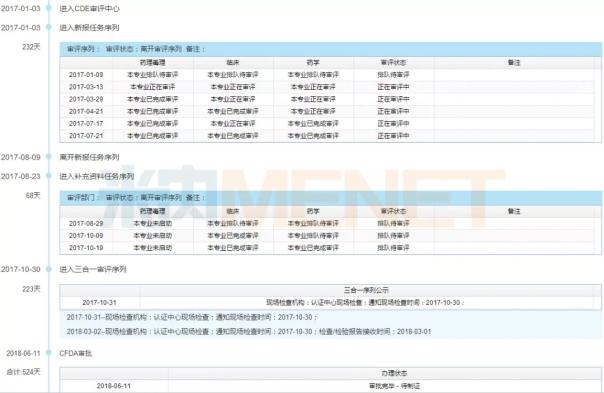
(Source: Minenet MED Drug Review Database 2.0)
In May of this year, the song officially submitted an IPO application to the Hong Kong Stock Exchange. In the new IPO regulations of Hong Kong, one of the conditions for allowing unprofitable biotech companies to go public in Hong Kong is to list the listed biotech companies at least 6 before the proposed listing date. A considerable amount of third-party investment is obtained in a month, and at least one core product has passed the concept development process. For the songs, Gonower's listing is undoubtedly a strong support for the company's IPO.
Sang Guowei, vice chairman of the Standing Committee of the 11th National People's Congress, academician of the Chinese Academy of Engineering, and chief technical officer of the National Major New Drug Creation Technology, said: "I am very pleased that China's first indigenous original hepatitis C innovative drug Gonowo was approved for listing. This is the country. A major achievement in the major new drug creation technology project shows that the innovation capacity of Chinese pharmaceutical companies is constantly strengthening and making breakthroughs in the field of major disease prevention and control."
The listing of Gonowei broke the monopoly of the domestic hepatitis C drug market by multinational pharmaceutical companies. Before the song, the anti-HC drugs of multinational companies such as Gilead, Aibowei, Bristol-Myers Squibb and Merck were approved. Although Gonower's time to market is a little late, if it can successfully enter the medical insurance catalog in the future, it may replace the original research products to seize most of the market.
Multinational companies have fought in China
5 drug companies fight, who will dominate?
Hepatitis C is a viral hepatitis caused by hepatitis C virus (HCV) infection. According to the World Health Organization's 2017 Global Hepatitis Report, about 325 million people worldwide are infected with chronic hepatitis B virus or hepatitis C virus. With the popularization and application of hepatitis B vaccine, the prevalence of hepatitis B has decreased. Compared with the prevention and control strategy of hepatitis B, there is no vaccine to prevent hepatitis C, and its incidence has shown a sharp upward trend. China is a "big country of hepatitis C", and cases of type 1, type 2, type 3 and type 6 account for 96% of all cases of hepatitis C virus, which promotes the continuous expansion of the hepatitis C drug market.
Table 1: Hepatitis C DAA drugs approved for domestic market from 2017 to the present
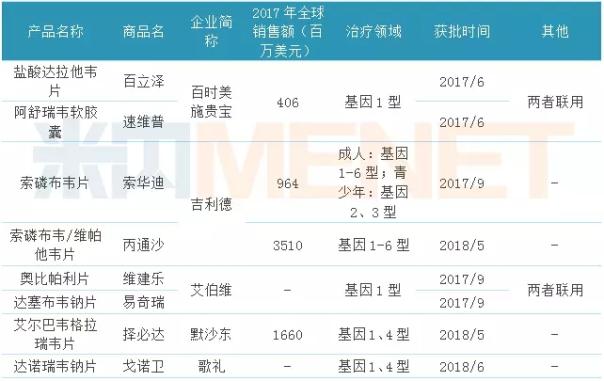
(Source: Minenet database)
Since 2017, the domestic hepatitis D DAA drugs have been approved for listing, which belong to Bristol-Myers Squibb, Gilead, Aibowei, Merck, and Song Li, among which the two drugs of Bristol-Myers Squibb and Aibowei are The combination of drugs, the listing of new drugs for songs and hepatitis C broke the monopoly of the domestic hepatitis C drug market by multinational pharmaceutical companies.
Gilead: Sophorabine tablets, sophobivox/ Vipatha tablets
Sophorabine tablets (trade name Sohuadi), a nucleoside polymerase inhibitor against hepatitis C virus, was launched in the United States in December 2013 and is suitable for partial hepatitis C genotyping. The cure rate is over 90%. Walt's global sales in 2017 was $964 million, a sharp drop from sales in previous years.
Figure 3: Global sales of Sophobs tablets in 2014-2017 (in millions of US dollars)
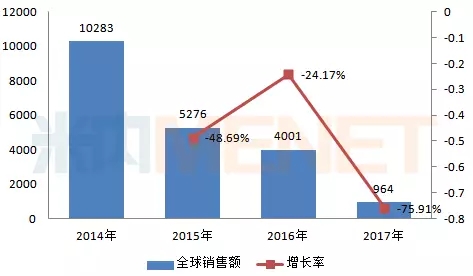
(Source: Minet's multinational listed company sales library)
In September 2017, Sohuadi was approved for listing in China. In November of the same year, Gilead announced that Sohuadi’s final price in China was 19,660 yuan/bottle, and the sales specification was 28 pieces/bottle, one for every three months. The course of treatment is calculated, the price of a course of treatment is 58980 yuan, and it is still a self-funded project.
Sophobvebuvir/Vipathavir (trade name: Bundongsha) is China's first approved pan-genotype, once daily, single tablet for chronic hepatitis C virus, suitable for genes 1-6 Type HCV adult patients. According to the data of five international multi-center phase 3 clinical studies, SVR12 (defined as the 12th after completion of treatment) in a patient group that is difficult to cure (including treated patients, and patients with compensated or decompensated cirrhosis) Week, the overall rate of detection of HCV RNA was 92%-100%.
Figure 4: Worldwide sales of Sophorabine/Vipatha tablets for 2016-2017 (in millions of US dollars)
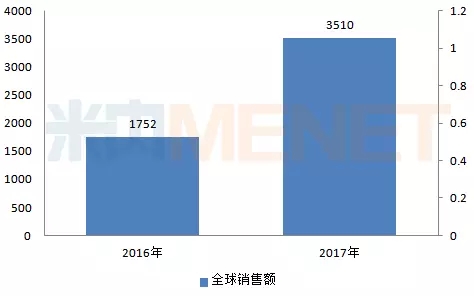
(Source: Minet's multinational listed company sales library)
Bundongsha was approved for listing in the US and EU in 2016 and has been approved by 54 countries. Its global sales in 2017 was US$3510 million, an increase of 100.34% compared with last year.
Bristol-Myers Squibb: Dalattavir Hydrochloride Tablets + Ashuruwei Soft Capsules
Daltavir hydrochloride tablets (trade name Bai Lize), an anti-hepatitis C virus NS5A inhibitor, ashuruiwei soft capsule (commercial name Weiweipu) is the second generation protease inhibitor developed by Squibb, Darah The complete oral combination therapy of Wei tablets and ashuvir capsules was approved in Japan in 2014, becoming the first fully oral, interferon-free treatment of hepatitis C genotype 1 patients in Japan, including decompensation. Clinical plan for patients with liver cirrhosis and hepatitis C. Global sales in 2017 were $406 million, a significant drop from last year.
Figure 5: Global sales of dalataxel hydrochloride tablets + arsulfide soft capsules in 2014-2017 (in millions of US dollars)
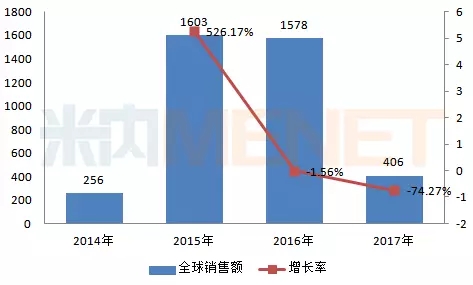
(Source: Minet's multinational listed company sales library)
After the approval of the “Ashuruwei†+ “Dalatavir†combination of Bristol-Myers Squibb in June 2017, it immediately launched a “pay-as-you-go†innovative insurance project in cooperation with Shanghai Pharmaceutical and Insurance. And other marketing efforts. According to the clinical study results of patients admitted to mainland China, the combined treatment regimen has a cure rate of 91% to 99% for patients with type 1b chronic hepatitis C, with good safety and tolerability.
Aibowei: Obi Pali + Dase Bude
Obi Pali film (trade name Wei Jianle) is an anti-hepatitis C virus combination preparation consisting of three kinds of drugs: amphetvirin, paclivir and ritonavir. Disebovir tablets (trade name Yi Qirui) It belongs to non-nucleoside polymerase inhibitors. Wei Jianle and Yi Qirui's program has been approved in more than 70 countries and regions around the world for the clinical treatment of patients with genotype 1 chronic hepatitis C.
Wei Jianle and Yi Qirui's treatment regimen consists of three direct antiviral drugs, namely NS5A inhibitor, NS3/4A protease inhibitor and NS5B polymerase non-nucleoside analogue inhibitor, which can target three major life cycles of hepatitis C virus. Target, inhibits the replication of hepatitis C virus. In September 2017, the treatment plan was approved for domestic marketing and was approved by the State Drug Administration in March 2018. The course of treatment was shortened from the original 12 weeks to 8 weeks.
Merck: Elbavir Grarewe
Albavir gragrevir tablets (brand name: Bida), a fixed-dose combination tablet consisting of erbawei and glarevir, using a one-week, 12-week course of treatment for gene 1, 4 or initial treatment Treatment of patients with recurrent chronic hepatitis C. Global sales in 2017 were $1,660 million, an increase of 199.1% from the previous year.
Figure 6: Global sales of Elbavir Grarewe in 2016-2017 (in millions of US dollars)
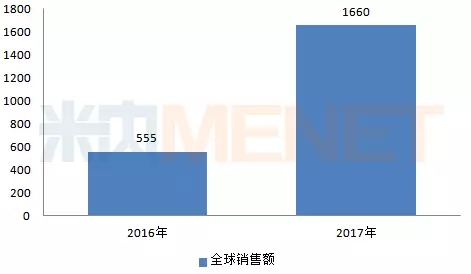
(Source: Minet's multinational listed company sales library)
Song: Danonevir sodium tablets
Danonevir sodium tablets (trade name: Gonowei) is a new generation of NS3/4A protease inhibitors developed by Songli with independent intellectual property rights. The results of phase III clinical trials completed in mainland China show that after 12 weeks of treatment, The cure rate (SVR12) was 97% in patients with genotype 1 non-cirrhosis; the cure rate was 100% in patients with genotype 4 non-cirrhosis.
Wu Jinqi, the founder of the song ceremony, once said: "If Gonowei's new drug certificate is approved today, I promise that Chinese hepatitis C patients will be able to cure hepatitis C after 21 days." Song Li can really use Chinese medicine for 21 days. Eat danovir sodium tablets? Let us wait and see.
In addition to the approved Gonowei, another anti-HCV class 1 drug, Ravidavir (a new generation of whole-genotype NS5A inhibitor), has also completed Phase II/III clinical studies. Clinical studies have shown that The first Chinese original full oral interferon-free regimen consisting of Vidawei and Gonower® has a cure rate (SVR12) of 99% in patients with genotype 1 non-cirrhosis, and for patients with NS5A resistance mutations at baseline, 100% implements SVR12.
Competition is heating up
11 hepatitis C drugs will accelerate listing
Table 2: Hepatitis C drugs that have been included in the priority review
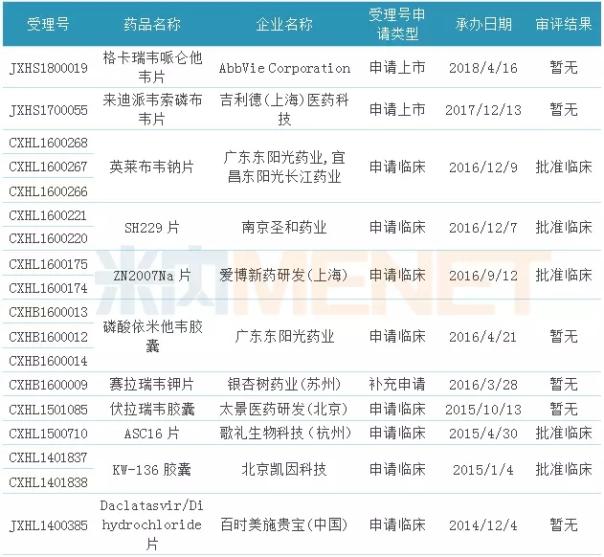
(Source: Ménet MED Drug Review Database 2.0, as of June 20, if there is any omission, please correct me)
According to the MED Drug Evaluation Database 2.0 data of Minernet, in the products that have been included in the priority review, there are 11 hepatitis C drugs currently in the application for clinical/application listing, including 3 imported drugs, involving Gilead. Aberdeen and other multinational pharmaceutical companies, including Gilead's Lai Di Wesom Phosphorus tablets and Aibowei's Gekarevir Peloxivir tablets are currently on the market. Eligibility for priority review of these hepatitis C drugs will help accelerate the R&D process and speed to market.
In addition, according to the data of the global drug research and development database of the intranet, domestic enterprises such as Taijing Biotechnology, Ginkgo Pharmaceutical, Changzhou Yusheng Pharmaceutical, Beijing Ruiyunhai Biological, etc. all have hepatitis C drugs in different research and development stages, and the future domestic hepatitis C market Competition is getting fiercer.
Dual Lens Flood Light Camera,Wireless Wifi Cctv Camera,1080P Camera,Security Surveillance Camera
Shenzhen Zuomi Technology Co., Ltd. , https://www.zuomicamera.com
